Adaptive Virtual Tutor for Chemistry Study Preparation 2 (VirtuTutor 2)
Beginner chemistry students need a lot of practice to internalise basic laboratory operations and routines. To aid with this problem, past project groups developed the VirtuChemLab. In this virtual reality application, students can practice in a safe environment which is independent from available lab space and independent from the availability of advisors. It is a project by students for students.
About
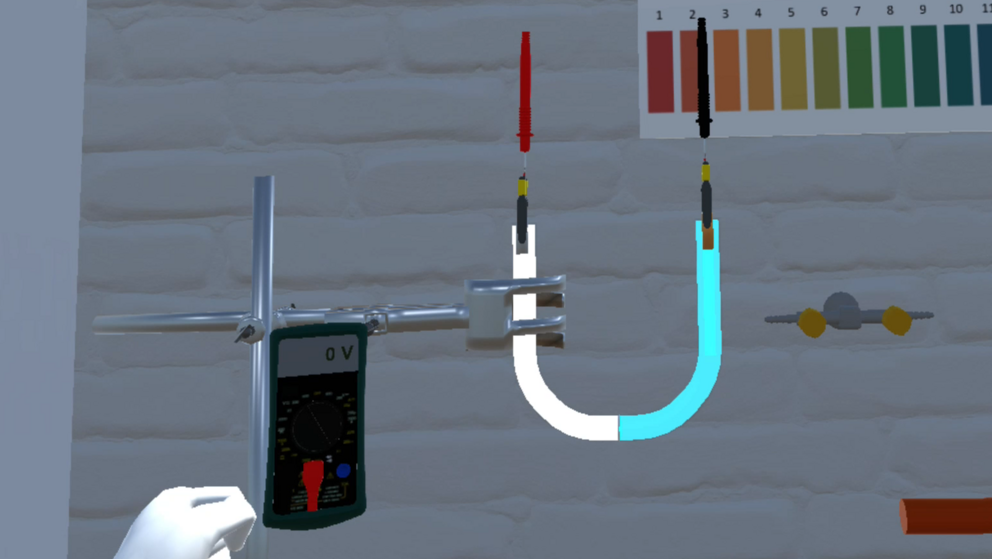
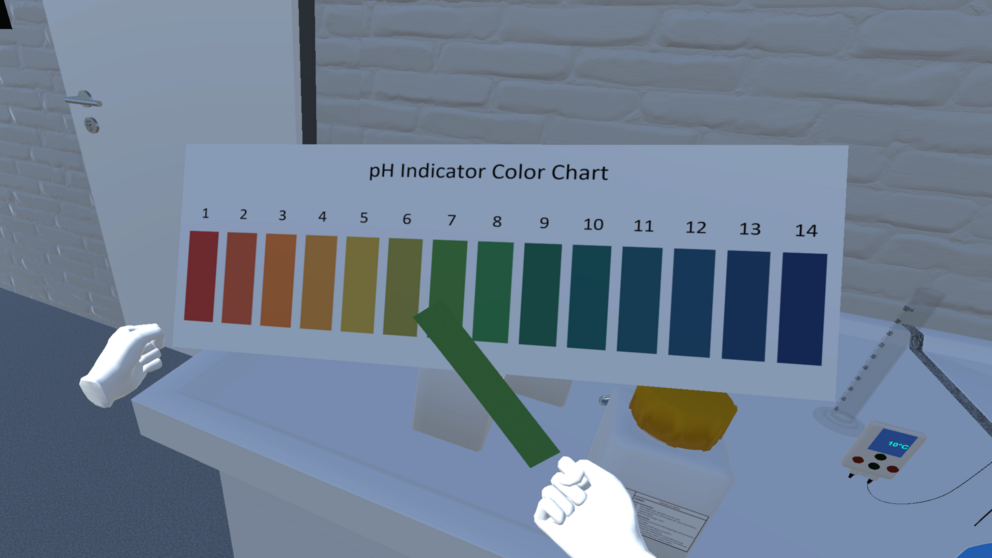
An example experiment
A virtual tutor for the VirtuChemLab
As one can see in the example above, we already have the basis of a virtual tutor. In this project group, we want to expand on this idea. For this, we want to focus on three main points:
- Assess the performance of students
- Have the tutor give positive and negative feeedback at appropriate times
- Learn to adjust the tutor’s behaviour based on student’s needs and performance
Below, you can see how this will translate to our example from above.
Our vision
Scope of the project group
For now, we aim to make the virtual tutor ready for two or three experiments in the VirtuChemLab. All of these experiments are already fully implemented, what needs to be done is to adjust the tutor accordingly.
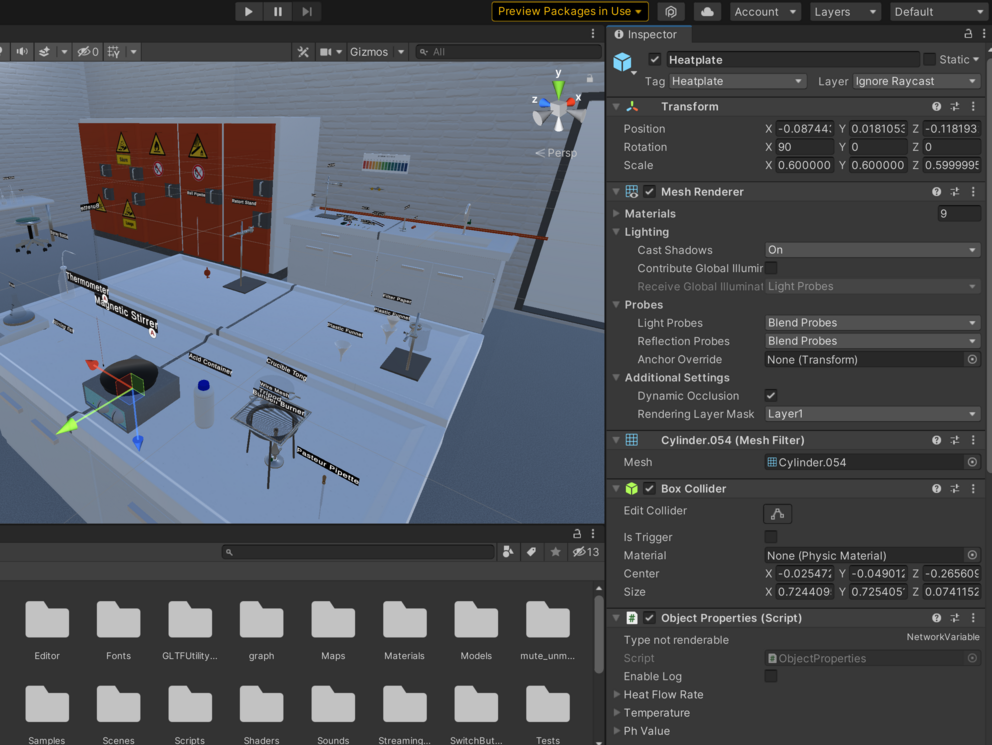
What we have
Our last project group already laid the foundations of the virtual tutor. Hence, things like speech input and output and rudimentary movement and animation of the tutor is already implemented. Furthermore, the group introduced rigurous logging of all events in the VirtuChemLab such that the tutor can observe what is happening in order to react properly. Additionally, the group also worked on having the tutor read the experiment instructions and intervene upon the detection of errors, e.g., when a students leaves a chemical container open for too long.
What we want
We want to improve the current state of the tutor by making it much more adaptable. This means that the tutor should closely monitor the actions of a student, grade their skills and adapt the tasks and reponses accordingly. For example, a new student might need much more help to assemble the gas burner. In that case, the tutor should notice that and give detailed instructions on how the burner needs to be set up. Analogously, when the tutor knows that the student is good at setting up the gas burner, it should give fewer hints as to not annoy the student.
Implementation
Interdisciplinarity

What is your role in the project group?
The chemistry didactics group of Prof. Fechner is very much interested in the VirtuChemLab and how it can improve teaching chemistry students in their first semester.
Since programming is not our forte, we act as a form of customer to the project group students which can help us to realize our ideas. I always found this cooperation to be very fruitful. I also think that it’s very intersting to the students as – like work in the industry – the customers aren’t computer scientists.
What level of chemistry knowledge do you expect from the students?
None! Both me and my colleague Marvin are always eager to assist with any chemistry-related questions which might come up. Hence, the project group students do not need any knowledge about chemistry.